Guidelines for COVID-19 vaccination of children between
15-18 years ,
India’s National COVID Vaccination Program is built on scientific and epidemiological evidence, WHO guidelines and global best practices. Anchored in systematic end-toend planning, it is implemented through effective and efficient participation of States/UTs and the people at large.

Government of India’s commitment to the vaccination program has been unwavering and proactive from the beginning, from strengthening Research and Development capacity, to encouraging and enabling manufacturing and vaccinating each and every adult Indian safely, as fast as possible. As a consequence of reliance on scientific & epidemiological evidence and pro-active implementation, India’s COVID-19 vaccination programme has achieved historical milestone of administering more than 141 crore doses so far. 90% of the adult population of the country has been covered with at least one dose and 62% of the adult population has been covered with both the doses.
For the COVID vaccination program, Government of India initiated early and proactive
steps as far back as April 2020:
• “Task Force for Focused Research on Corona Vaccine” (constituted in April
2020), to encourage domestic R&D of Drugs, Diagnostics and Vaccines, headed
by Principal Scientific Advisor to the Government of India.
• “National Expert Group on Vaccine Administration for COVID-19” (NEGVAC),
(constituted in August 2020), to formulate a comprehensive action plan for
vaccine administration, co-chaired by Member (Health) NITI Aayog and Union
Health Secretary.
• “Empowered Group on Vaccine Administration for COVID-19” (constituted in
January 2021), to facilitate optimal utilization of technology to make COVID
vaccination all inclusive, transparent, simple and scalable, headed by CEO,
National Health Authority.

India’s COVID vaccination program incorporates recommendations of the foremostexperts in the field of immunization, public health, disease control and information technology. Based on scientific and epidemiological evidence, the programme gives priority to strengthening the country’s healthcare system by protecting the professionals, health and frontline workers, manning it, as well as protecting the most vulnerable population groups.
COVID vaccination in the country commenced with vaccination to all Health Care Workers. The program was expanded with time to include vaccination of Front Line Workers, citizens more than 60 years of age, citizens more than 45 years of age, and eventually citizens more than 18 years of age.
Under the National COVID Vaccination Program, from 16th January to 30th April 2021,100% of vaccine doses were procured by Government of India and provided free ofcost to State Governments. State Governments were in turn to administer vaccinationfree of cost to defined priority groups. To increase the pace of vaccination,participation of private hospitals was also enlisted where individuals could also choose to get vaccinated at a prescribed rate.
In response to the suggestions of many State Governments to be permitted the
flexibility to procure vaccine directly and administer them as per their own
prioritization based on local requirements, Government of India revised the
Guidelines. Under the revised Guidelines effective from 1st May, 2021, Government of India was procuring 50% of the vaccine produced and was continuing to provide them to States free of cost for administering to priority groups. The State Government and private hospitals were also empowered to directly procure from the remaining 50% vaccine pool.
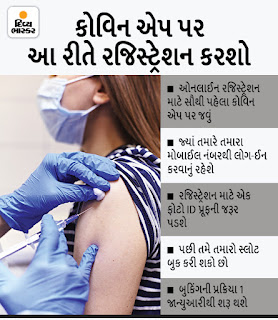
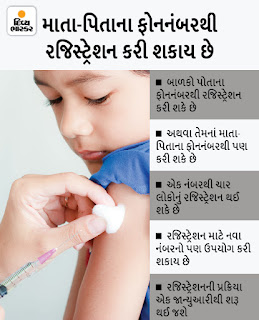
Vaccination slots can now be booked for children aged 15-18 yrs (CLICK HERE)
Many States subsequently communicated that they were facing difficulties in
managing the funding, procurement and logistics of vaccines, impacting the pace of the National COVID Vaccination Program. It was also noted that smaller and remoter private hospitals also faced constraints. Keeping in view the aforesaid aspects, the experiences gained from 1st May 2021 and the repeated requests received from States, the Guidelines for National COVID Vaccination Program were reviewed and revised. These Revised Guidelines became effective from 21st June 2021. Under the Revised Guidelines, Government of India procured 75% of the vaccines being produced by the manufacturers in the country and provided it free of cost to States/UTs as has been the case from the commencement of the National Vaccination Programme.
These doses were administered by the States/UTs free of cost to all citizens as per priority through Government Vaccination Centres. Vaccine doses provided free of cost by Government of India have been allocated to States/UTs based on criteria such as population, disease burden and the progress of vaccination. Wastage of vaccine has affected the allocation negatively.
How Safe Corona Vaccine for Your Baby????? (CLICK HERE)
ALSO READ THIS:-



